Philips Heartstart FR3 Defibrillator




Philips Heartstart FR3 Defibrillator
0 item is in your cart. View cart now
All product and company names are trademarks of their respective holders. Use of them does not imply any affiliation with or endorsement or sponsorship by them.
Details
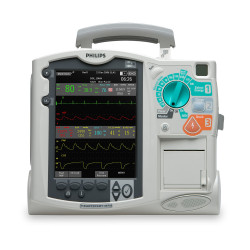
The Philips Heartstart FR3 Defibrillator is the smallest, lightest professional-grade defibrillator / AED available from leading global manufacturers. Weighing just 3.5 pounds, the Heartstart FR3 can be easily loaded into a carrying case and transported with other emergency medical equipment.
Designed to allow emergency technicians to work quickly in the field, the Philips Heartstart FR3 features pre-connected SMART PAD III’s, Philips SMART CPR guidance software, a high-resolution color LCD screen, and is available in a bilingual configuration.
The Philips Heartstart FR3 Defibrillator / AED is available professionally refurbished from Avante Health Solutions. For more information, contact an Avante representative today.
Features
- Small, lightweight, professional-grade AED ideal for mobile use.
- Unit automatically initiates when the carrying case is opened, allowing emergency professionals to focus on pad placement.
- Pre-connected SMART PADS III don’t have foil pouches, just peel and place.
- Patient-specific guidance with Philips SMART CPR.
- Philips Quick Shock technology minimizes CPR interruptions.
- Weighs only 3.5 pounds and is available with a wide array of carrying case options for easy transport.
- Durable AED is tested to US Military standards and can withstand demanding work conditions.
- AED performs periodic, automated self-tests to help ensure proper function.
- Bilingual configurable so that voice and text prompts can be clearly understood.
- High-resolution color LCD for easy viewing in any environment.
- Optional — Infant/Child key automatically decreases defibrillation therapy in accordance with pediatric CPR protocols.
Specifications
Defibrillator
- 861388 text display
- 861389 ECG and text display
- Waveform: SMART Biphasic Truncated Exponential waveform parameters adjust as a function of patient impedance. Adult nominal peak current 32A (150J into a 50 ohm load): pediatric nominal peak current 19A (50J into a 50 ohm load) using optional Infant/Child Key
- Shock delivery: Via defibrillator pads placed in the anterior-anterior(Lead II) position for adults; anterior-posterior position for infants and children under 55 lbs or 8 years old
- Controls: On/Off button, shock button, option buttons. Auto-On feature, when used with the optional FR3 carry case, enables FR3 to power up when case lid is opened
- Indicators: High-resolution color LCD, beeper, voice prompts, tones and chirps, audio speaker, connector socket, ready light, shock button
- Advanced mode: Configurable using optional HeartStart Configure software
ECG Display
- Screen: LCD color display, 320 x 240 pixels. 2.8" x 2.1"
- 1 Hz to 30 Hz (-3dB), nominal (non-diagnostic)
- Lead II using anterior-anterior adult pads placement
Physical
- Size: 2.7" high x 5.3" wide x 8.7" deep
- Weight: 3 lbs 8 oz with FR3 primary battery installed
Environmental
- Sealing: Meets IEC529 class IP55 with battery installed
- Temperature: Operating/standby: 32°–122°F
- Altitude: Meets IEC 60601-1:5.3 (1013 to 572 mbar (hPa), equivalent to air pressure from 0 to 15,000 feet)
- Shock/drop Abuse tolerance: Meets MIL-STD-810F 516.5, Procedure IV (after a one-meter drop to any edge, corner, or surface in standby mode)
- Vibration: Meets MIL-STD-810F 514.5 C-17
Bluetooth 2.0
- Bluetooth 2.0 Class II Wireless Transceiver Module
- Function: Transmit retrospective event data or configuration setting wirelessly
Patient analysis system
- ECG analysis: Evaluates impedance of defibrillator pads for proper contact with patient skin, evaluates the ECG rhythm and signal quality to determine if a shock is appropriate; also detects artifact and pacemaker
- SMART CPR: Evaluates key characteristics of the presenting VF and determines the initial therapy: shock first, or CPR first quickly followed by a shock
- Sensitivity/specificity: Meets AAMI DF80 requirements and AHA recommendations for adult defibrillation
- Quick shock: Typically arms in <8 seconds from the end of the “Stop CPR” prompt
FR3 primary battery
- Type: 12 VDC, 4.7 Ah, lithium manganese dioxide | Long-life primary cells
- Typically 300 shocks or 12 hours of operating time at 77° F (25° C) when configured for monitoring after No Shock Advised (NSA) | 7.5 hours of operating time at 77° F (25° C) when configured for CPR after NSA
- Standby life: 3 years minimum when stored under standby environmental conditions (battery installed)
- Shelf life: 5 years
SMART Pads III
- Application: Disposable, multifunction defibrillation pads for adult or infant/child patients. Time-saving peel and place pads can be removed from packaging and stored in the FR3 carry case. Pads can be preconnected to FR3, which enables testing during FR3’s routine self-test.
FR3 data card
- Function: Stores a minimum of 8 hours of ECG, event, and, if configured, voice recording. Can also be used for configuring FR3
Automated and user-activated self-tests
- Automatic self-test: Tests internal circuitry, waveform delivery system, ECG acquisition, temperature, status (or readiness) of attached accessories (SMART Pads III and FR3 data card) and battery
- Automated self-test frequency: Daily, weekly, monthly, power on, and runtime during all modes of operation
- User initiated tests: Automatic self-tests plus tone, display, and button performance
FR3 training battery and training pads (optional)
- Function: Places FR3 into a scenario-based training mode and simulates shock therapy
- Type: 10.8 Volt, 4.5 Ah Li-ion battery